WESF plays a role in promoting standardization and eliminating global trade barriers.
Incubators need to have power at all times.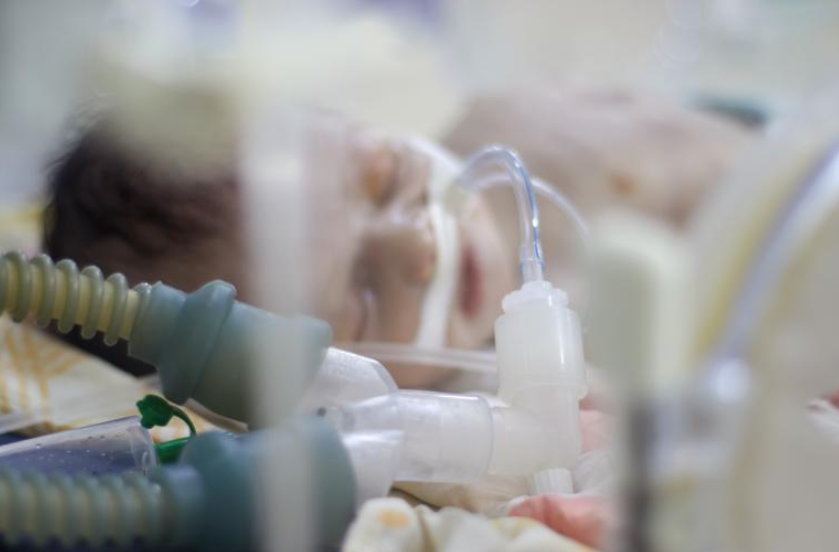
Since the mid-19th century, the earth’s average surface temperature has risen by almost 1,5 °C. This change – driven largely by increased carbon dioxide emissions into the atmosphere, alongside other detrimental activities caused by humans – is causing extreme weather events to occur on a more frequent basis than ever before, from floods to droughts.
These changing weather patterns are having an effect on healthcare systems around the world. Hospitals in developed countries have built-in redundancy systems to enable them to continue working even if there are power cuts.
These hospitals generally have on-site generators that provide overall power backup for extended periods, as well as battery-powered uninterruptible power supply (UPS) systems for instant power to ensure continuous and stable electricity for critical safety systems, from life-saving patient devices to computers.
In developing countries that regularly suffer from unreliable electrical power supplies, there tends to be a general lack of resources to implement such systems. According to the World Health Organization (WHO), close to one billion people in low- and lower-middle-income countries are served by healthcare facilities with unreliable electricity supply or with no electricity access at all. While some hospitals have standby generators, they often lack the fuel needed to operate them. Having enough electricity to work in normal conditions – let alone when impacted by severe weather – is already a challenge.
In the developing world, a UPS is a vital line of defence – whether as a general UPS, or an in-device UPS – to prevent disruptions, and even death. UPS systems ensure that medical devices in hospitals, through to the computers and communications systems used by staff, keep working when the power grid fails.
IEC Standards are key to ensuring that everything – from transformers and substations to electrical lines, and more – operates safely and efficiently. A wide range of these standards covers the generation, transmission and distribution of electricity, and many of these ensure that the grid can either keep running or get back to normal quickly after an extreme weather event has generated disruption. (For more on this, read: Building resilience into the grid | IEC e-tech).
The safety and performance of battery cells are specified by IEC TC 21 standards. The standard most referred to for medical devices is IEC 62133-1. This standard sets the requirements for lithium-ion battery safety in devices used in healthcare, robotics, security systems, infrastructure, and consumer electronics.
More specifically, IEC Standards relating to medical devices ensure that redundancy and failure prevention features are built in. The IEC technical committee which prepares these standards, IEC TC 62, has recently embarked on the revision of one of its core standards, which outlines general requirements for the safety and essential performance of medical electrical equipment, IEC 60601-1.
This fourth edition of IEC 60601-1 places software and connectivity – including artificial intelligence (AI) – as the key pillars of medical safety. Regina Geierhofer, Secretary of IEC TC 62, explains: "What we're doing now in the fourth edition, among many things, is tightening the safety requirements because you have to assume that power outages will be more common under the new [climate change] weather circumstances."
Before IEC TC 62 Standards come into play, there are many layers of protection for hospitals to ensure a reliable power supply. According to Geierhofer, "The medical devices are the last line of defence." She adds: "If all the paths before break, then medical devices will still, when they meet essential performance requirements, work for a certain time; not forever, but for a certain time, so no patient is immediately endangered."
UPS systems for hospitals are vital, and for essential life-saving and life supporting devices, an internal UPS is required. "You can imagine essential performance machines," says Geierhofer. "Let's say you have a machine which breathes for you during a surgery, because you cannot breathe yourself. Now if the power is no longer there, and even if the hospital does nothing to reestablish it, the medical device itself has an internal power supply. Usually, it's not used, but in the moment the devices have no external power supply, the internal one – a battery – usually kicks in. But we all know there is a limit to a battery as it needs recharging. Therefore, all medical devices need to meet certain safety requirements that we specify in our standards.”
In addition, due to various issues such as budget cuts and the increasing numbers of devices coming into the hospital ecosystem, a UPS may not have enough capacity to be able to handle all the devices drawing from it in the event of a power outage. Geierhofer comments: "Having a UPS that can support all of what has to be supported can be a challenge for the hospital."
IEC 60601-1 specifies that a device must remain safe and perform its essential functions even when a single component fails or an abnormal condition occurs. The idea is that patient safety and user safety are maintained. It therefore requires manufacturers to design devices with a low probability of failure or include various mechanisms to control risk.
An added safety level lies with conformity assessment, which can help manufacturers meet the requirements established in standards. IECEE, the IEC System of Conformity Assessment Schemes for Electrotechnical Equipment and Components, offers testing and certification for the safety, reliability, efficiency and overall performance of electrical equipment for medical use to IEC International Standards, whether new or refurbished.
The IECEE Testing Laboratories (CTL) Expert Task Force covers two areas:
measurement, control and laboratory equipment related to the IEC series of standards IEC 61010 for safety requirements for electrical equipment for measurement, control, and laboratory use
electrical equipment for medical use related to the IEC 60601 series of standards for the general requirements for basic safety and essential performance of medical electrical equipment and medical electrical systems.
The last line of defence is therefore being reinforced for the benefit of medical staff and patients.